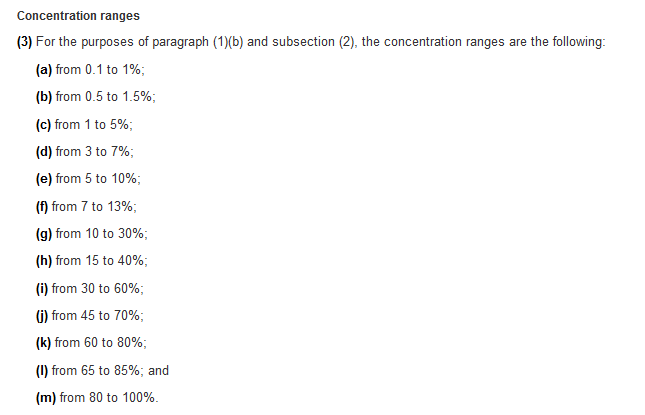Canadian Safety Data Sheet Requirements: Disclosure of Ingredients
By: Alison Senyi, SENIOR PRODUCT SAFETY SPECIALIST, email
Which information is required on an SDS in Canada? We get asked this question often. If your company is bringing a workplace product from the United States, Asia or Europe, it’s an answer you will need in order to be compliant in Canada.
With over 40 years of experience with authoring safety data sheets for Canada and the US, the team at Dell Tech can help you.
What is an SDS?
Safety Data Sheets (SDSs) are documents that show the hazards of a product and give information on the related safety precautions. Safety data sheets include information such as:
- A product’s health, physical, and environmental hazards;
- emergency measures;
- physical and chemical properties of the product; and
- safety precautions when handling the product.
Safety data sheets are an important resource in workplaces to inform the workers of the products being used. SDSs tell the user about the product hazards and how to use the product safely, any first aid requirements, emergency procedures to follow, along with personal protective equipment to use during exposure.
A safety data sheet provides more detailed hazard information than the label of the product.
Note: Although it is not common, you may still see these documents referred to as material safety data sheets. It’s an outdated term but refers to the same type of document.
Why do I need an SDS in Canada?
Any product that has been classified as hazardous under the Workplace Hazardous Materials Information System (WHMIS) regulations, and that will be used, stored and handled in a Canadian workplace needs to have an SDS. In order to meet official language requirements of Canada, the SDS must be available in both English and French. One bilingual SDS, or two separate language safety data sheets are acceptable.
What information is required on a Canadian SDS?
The Hazardous Products Regulations (HPR) outlines the sections and information required to be on the SDS. Schedule 1 of the HPR shows the section numbers and headings that must be present. The 16 sections and corresponding headings of the Canadian SDS are as follows:
- Identification
- Hazard identification
- Composition/Information on ingredients
- First-aid measures
- Fire-fighting measures
- Accidental release measures
- Handling and storage
- Exposure controls/Personal protection
- Physical and chemical properties
- Stability and reactivity
- Toxicological information
- Ecological information
- Disposal considerations
- Transport information
- Regulatory information
- Other information
*Note: according to the Canadian regulations, sections 12 to 15 of the SDS only require the headings to be shown. Including any information in these sections is optional.
What are the requirements for Section 3 of the Canadian SDS?
The disclosure of chemicals in section 3 of the SDS can be difficult to follow. The requirements for disclosure on a Canadian complaint SDS are outlined in Schedule 1 of the HPR guidelines. You must include the chemical name/common name, CAS number, and concentration. If you choose NOT to include the CAS number or proper chemical name, then you must apply for a HMIRA Trade Secret.
Throughout the years, the information on how to show the concentration on an SDS has changed. In the older Controlled Products Regulations, there were a set of ranges that Health Canada permitted to use on an SDS. Once the Globally Harmonized System (GHS) (WHMIS, 2015) was implemented, these ranges could no longer be used, and exact percentages were required.
An amendment to the Hazardous Products Regulations (HPR) came into effect in 2018, and the use of prescribed concentration ranges on SDSs was once again permitted if the actual concentration ranges were withheld as trade secrets. This new set of concentration ranges was not the same as what was used in the past.
See information below for the HPR concentration ranges:
HAZARDOUS PRODUCTS REGULATIONS:
What is the difference between Canadian and US SDSs?
There are a few differences between the Canadian SDS and labelling requirements and those for the US. These include the use of prescribed concentration ranges in accordance with the HPR; adding the Canadian supplier address information on SDSs and labels; the requirement to update SDSs and labels whenever suppliers are made aware of ‘significant new data’; hazard classes such as ‘Physical Hazards Not Otherwise Classified’ and ‘Health Hazards Not Otherwise Classified’; and the requirement of bilingual (English and French) SDSs and labels.
Dell Tech can assist you with an SDS unique to Canada or the United states, or provide you with a combined SDS that will be compliant in both countries.
How can Dell Tech help with my SDS needs?
Please contact our Product Safety team for further information on your SDS requirements for Canada.
Our services include:
- SDS authoring for Canada, US or combined
- Technical translations for safety data sheets and chemical labels
- Updating existing documents to be in compliance with the Hazardous Products Act
For a full list of Dell Tech’s safety data sheet services, visit our SDS service page.