Understanding Plain Language Labelling for Canadian Natural Health Products (NHPs)
By: Karolina Zarichna, REGULATORY, BUSINESS DEVELOPMENT & MARKETING SPECIALIST, email
Overview of the Upcoming Changes for Natural Health Product Labels
In 2022, Health Canada implemented an update to the labelling regulations for Natural Health Products. The update unveiled a standardized labelling approach modelled after the drug product labelling requirements. This amendment introduces the Product Facts Table (PFT), structured to streamline product information into a table format. The primary objective of the PFT is to offer consumers a clear and accessible layout for retrieving product-related details.
Alongside the introduction of the standardized PFT, Health Canada enacted revisions for allergen labelling, technical specifications, and declaration of contact information.
Learn more about the upcoming changes in our post below.

General Labelling Requirements for Natural Health Products
Labels for Natural Health Products typically consist of two primary sections: (1) the principal display panel; and (2) other areas, which includes any part of the outer label except for the bottom. Natural Health Product Regulations dictate what information must appear on the product label and mandate that it reflects the issued product licence. Mandatory product information must appear in both official languages, English and Canadian-French, in the same prominence. For support with professional label translations, please visit our Technical Translations for Labels & Safety Data Sheet service page.
Additionally, certain products feature both inner and outer labels. For instance, a natural health product may have an inner label on the bottle and an outer label on the box that it’s sold in. While the principal display panel requirements remain consistent, the required information on other label areas may vary between outer and inner labels.
Principal Display Panel (PDP) Labelling Requirements
An NHP’s principal display panel, also known as PDP, is the label that faces the consumer at the time of purchase and is visible under normal conditions of use. In other words, this is the front-facing panel of the label when it is sitting on a store shelf. The following information must be included on the PDP and be visible to the consumers at the time of purchase:
- Brand name – A product’s brand name must be included on the PDP of the product and correspond to the information listed on the product licence. If a specific brand name is not listed on the licence of your product, it cannot appear on the label.
- Natural Product Number – An eight digit natural product number issued by Health Canada must appear on the PDP and include an introductory prefix “NPN”. This number should be displayed horizontally on the panel.
- Dosage form – The product’s dosage form refers to how the product is applied or its form. For example, a dosage form can be a “tablet” or a “capsule”. If the dosage form of the product is indicated in the product’s brand name, it does not need to be repeated again.
- Net amount – The net amount is the total count of the dosage unit in the immediate container or the total amount of contents within, declared using a metric system.
- Sterile (if applicable) – Products that are sterile must contain a “sterile” callout on the PDP, appearing in both English and French.
Other Labelling Requirements
The labelling criteria for additional sections of the product label vary depending on the type of product label. Distinctions in labelling requirements exist for products featuring both an inner label (attached directly to the container holding the contents) and an outer product label (enclosing the product’s container, like an outer box). If your product contains only one label (inner label only), then it must comply with the labelling requirements of the outer label:
Outer Label Requirements: The information to be included on other label panels, in addition to the the principal display panel requirements, includes:
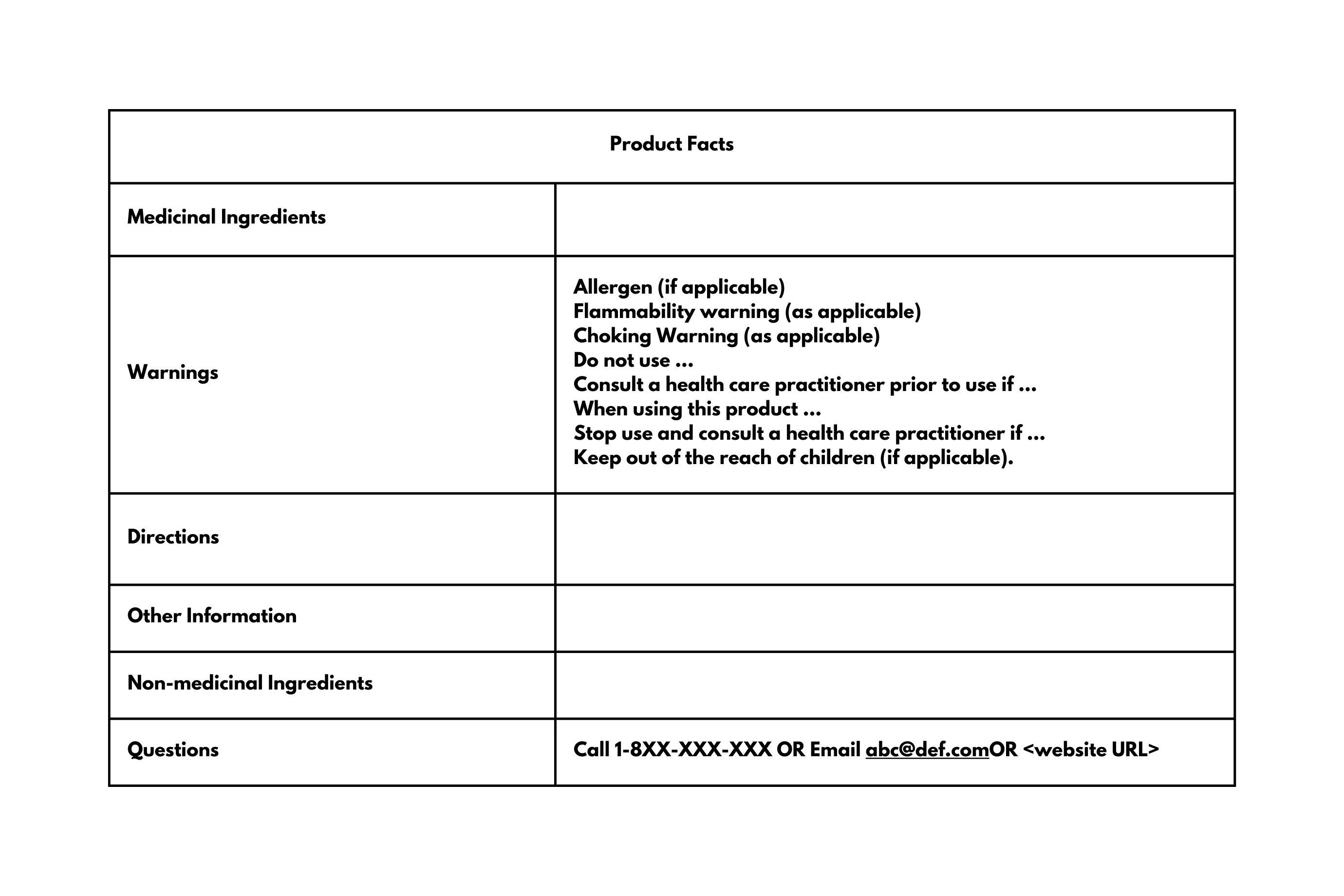
- A Product Facts Table, encompassing: the list of medicinal ingredients, uses or purposes (i.e., health claims), warnings (warning statements, cautions, contraindications, known adverse reactions, directions of use (including recommended dose and frequency), allergen, and related statements), other information (storage conditions or special instructions), non-medicinal ingredients, and a “Questions?” section.
- The name of the licence holder (as stated on the product licence) or the importer’s name.
- Recommended route of administration, such as “oral”, “nasal”, “sublingual”, etc.
- Lot number, prefaced with an appropriate identifier like “Lot number” or “Lot No.”.
- Expiry date, prefaced with an appropriate identifier such as “Expiry” or “EXP”. Various declaration formats are permissible, including EXP YYYY-MM or EXP MM-YYYY.
Inner Label Requirements: Products featuring both outer and inner labels must adhere to the following labelling requirements for their inner labels:
- The name of the licence holder (as it appears on the product licence) or the importer’s name AND their contact information.
- The list of medicinal ingredients, their quantity per dosage, and potency, where applicable.
- At least one use or purpose (as specified on the product licence).
- Recommended route of administration, for instance, “oral”, “nasal”, “sublingual”, etc.
- Dosage.
- Duration of use.
- Allergen statement(s) and indication of its source material, such as food allergens.
- Risk information.
- Other information (storage or special instructions).
- Lot number, prefaced with an appropriate identifier such as “Lot number” or “Lot No.”.
- Expiry date, prefaced with an appropriate identifier like “Expiry” or “EXP”. Various declaration formats are permissible, including EXP YYYY-MM or EXP MM-YYYY.
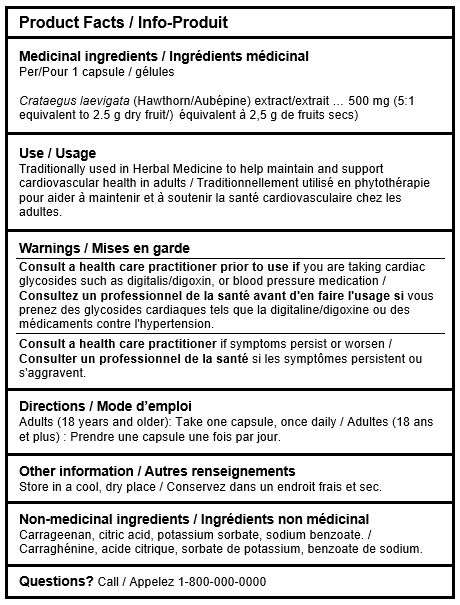
Product Facts Table (PFT) Information
The addition of the Product Facts Table (PFT) to the NHP regulations marks a significant change. Its goal is to present essential product details in a structured format. The table must adhere to the following order:
- Title: The title “Product Facts” should be prominently displayed in both English and French. Licence holders can choose between a bilingual table or separate English and French tables.
- Medicinal Ingredients: Details of medicinal ingredients should be provided and listed in the descending order based on the ingredient amount:
- Proper name of the ingredient and its source material.
- Quantity per dosage unit (e.g., per capsule).
- For extracts, include extract ratio and quantity crude equivalent.
- Potency of each ingredient (e.g., standardization).
- Uses and Purposes: Indications of use should follow medicinal ingredients, including at least one health claim from the product’s licence. Only claims from the product monograph or licence are allowed; marketing claims are prohibited.
- Warnings: All caution and risk statements from the market authorization or product licence should be listed in the following order:
- “For ______ use only” (if applicable).
- Allergen, gluten, and/or added sulphites statement (if applicable).
- Flammability warning (if applicable).
- Choking warning (if applicable).
- “Do not use” statements.
- “Ask a healthcare practitioner before use if” statements.
- “When using this product” statements.
- “Stop use and ask a healthcare practitioner if” statements.
- “Keep out of reach of children” statement.
- Directions: Directions for use, including suggested daily dose and duration, should be provided after the warnings section.
- Other Information: This section, placed after the directions of use, should include storage instructions or any other relevant product-handling information.
- Non-Medicinal Ingredients: Listed by their common names, non-medicinal ingredients should follow other information and can be listed alphabetically or by quantity.
- Questions: Lastly, a “Questions” section must be added after the non-medicinal ingredients, listing the contact information of the product licence holder.
 For additional information on Product Facts Table formatting, exemptions, and innovative labels please visit the Labelling of Natural Health Products Guidance Document.
For additional information on Product Facts Table formatting, exemptions, and innovative labels please visit the Labelling of Natural Health Products Guidance Document.

Compliance Deadline
All NHPs licensed before June 21, 2025, must follow the new Plain Language Labelling guidelines by June 22, 2028. For products licensed on or after June 21, 2025, compliance with the new amendments will be necessary upon licensing.
We’re Here for Regulatory Support
Unlock the full potential of your Natural Health Products with Dell Tech’s unparalleled expertise. With over 40 years of industry experience, our team of specialists is dedicated to supporting Canada’s thriving natural health product market. From licensing and testing to labelling and comprehensive consulting, we provide a complete suite of professional services tailored to your needs. Discover how Dell Tech can help you succeed. Visit our Natural Health Products service page today!
Dell Tech has provided professional, confidential consulting services to the specialty chemical
industry in Canada, the USA, Europe, and Asia for the last 40 years.





