2024 Supplemented Foods – What are they and how are they regulated?
By: Karolina Zarichna, REGULATORY, BUSINESS DEVELOPMENT & MARKETING SPECIALIST, email
About Supplemented Foods
With a compliance deadline of December 31, 2025, Supplemented Foods represent a newly introduced product category in the Canadian market, initially incorporated into the Food and Drug Regulations in 2021. This category includes prepackaged foods that contain supplemental ingredients, which are added to provide physiological or health benefits.
Learn more about the new regulatory framework and key highlights of the upcoming changes below.
Supplemented Food Regulatory Framework
On July 20th, 2022, Health Canada published a regulatory amendment, amending the Food and Drug Regulations and introducing a new food category – Supplemented Foods. The purpose of the amendment was to introduce and establish detailed conditions of use for supplemented ingredients in prepackaged foods, creating a predefined criteria and conditions of use that would no longer require a pre-market assessment.
The amendment introduced a List of Permitted Supplemental Ingredients and a List of Permitted Supplemented Food Categories, which outline what supplemental ingredients can be used in prepackaged foods and the food categories to which they can be added. In addition to outlining permitted ingredients, the List of Permitted Supplemental Ingredients provides an approved daily dosage for the listed ingredients and other conditions of use.
Regulations pertaining to Supplemented Foods are published in the Supplemented Food Regulations. However, additional considerations must be made for associated regulations, such as the Food and Drug Regulations and the Food and Drugs Act. This also includes any documents incorporated by reference, which are published outside of the applicable food regulations.
The applicable regulations are a responsibility of the manufacturer and the distributors. These parties are responsible for ensuring that all applicable regulations are met. This includes considerations of the following rules and regulations:
- List of Permitted Supplemented Food Categories
- List of Permitted Supplemental Ingredients and their conditions of use
- List of Permitted Food Additives
- Regulations for Food Flavouring Ingredients
- Novel Food Classifications
- Labelling Requirements
- List of Cautionary statements:
- Cautionary statement for supplemental ingredients (where applicable)
- Cautionary statements related to the number of servings (where applicable)
- Cautionary statements against use by certain population groups (where applicable)
- Cautionary statements related to use of other supplemented foods or supplements (where applicable)
- Principal display panel (PDP) statements (where applicable)
- The Supplemented Food Facts Table (SFFt) (more about the SFFt is included below)
- The Supplemented Food Cautionary Identifier (SFCI) (where applicable)
- Front-of-Package (FOP) Nutrition Labelling (where applicable)
- Food Labelling Requirements outlined in the FDR
- Priority Allergen, Gluten Sources, and Sulphites Labelling (where applicable)
- List of Cautionary statements:
Temporary Market Authorization (TMA) Transition to Supplemented Food Regulations
As some of the products in the Supplemented Food category have previously received a Temporary Market Authorization to sell their product in Canada, considerations have been made for their transition.
- Products that received a Temporary Market Authorization Letter (TMAL) expiring on July 21, 2022, appearing under Table 1, can be sold under the TMA conditions until the Supplemented Food Regulation compliance deadline of December 31, 2025. During this transition period, products with expired TMALs must adhere to the conditions and responsibilities specified in the issued authorization and associated regulations. However, by the compliance deadline, they must be transitioned to meet the new Supplemented Food Regulations. Additionally, during the transition period, these products must comply with majority of applicable Food and Drug Regulations that were in place before the introduction of the Supplemented Foods category and regulations. Exemptions from regulatory requirements stated in the TMAL apply throughout the transition period.
- Health Canada is still reviewing the TMA applications submitted prior to July 21, 2022. Applicants and responsible parties will receive a written notice authorizing the sale of their products given that they meet the transitional provisions. Applicants receiving a written notice will have until December 31, 2025 to ensure compliance with the Supplemented Food and associated regulations.
- For Supplemented Foods entering the market after July 21, 2022 must comply with the new regulations.
Please note, any changes to supplemented foods with a TMA will result in a loss of a transitional period. Meaning, introducing changes that fall outside of the authorization will require the manufacturers and distributors to ensure compliance with the new Supplemented Food Regulations immediately.
Information to be Declared Supplemented Food Labels
Supplemented foods are similar to regular foods and must adhere to the core labelling requirements specified in the Food and Drug Regulations. These requirements include listing ingredients, allergens, net quantity, common name, principal place of business, and other labelling declarations. Additionally, supplemented foods must include:
- A Supplemented Food Facts Table
- Cautionary statements (where applicable)
- A Supplemented Food Caution Identifier (SFCI) (where applicable)
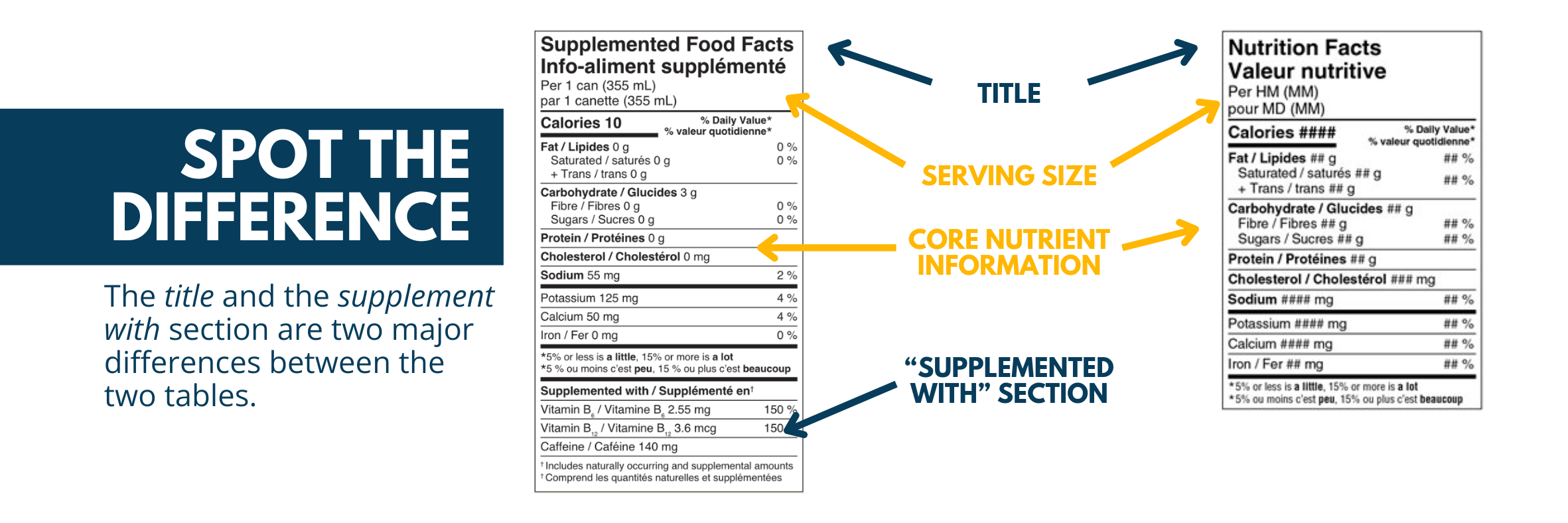
Supplemented Food Facts Table
The Supplemented Food Facts Table (SFFt) is a standardized labelling initiative similar to the traditional Nutrition Facts Tables found on regular food products. The key difference is that the SFFt includes an additional section that lists the supplemental ingredients added to the product, their amounts, and their applicable daily values (DV%). This feature allows consumers to easily identify supplemented foods and make informed decisions.
Several formats of the SFFt are available for manufacturers and distributors to use for their products, accounting for the size and shape of the food containers. This includes horizontal, vertical, and compact table layouts. A compendium of SFFts can be request from Health Canada to aid in product labelling.
It is important to note that all SFFt tables must follow the established technical requirements, which include type size, colour, and font. Additional considerations must be made for spacing requirements and background colours.
An example of the Nutrition Facts Table and a Supplemented Food Facts Table can be seen below.
Learn about regular Food Labelling Requirements here.
Supplemented Food Caution Identifier (SFCI)
In addition to a Supplemented Food Facts table, some products must include a Supplemented Food Caution Identifier (SFCI) on their principal display panel. An SFCI is necessary when the supplemental ingredients in the product require a caution statement.
For example, a carbonated energy drink with added caffeine requires a cautionary statement if the total amount of caffeine from all sources exceeds 150 ppm (0.015%). The label must include the caution statement: “Not recommended for those under 14 years old, pregnant or breastfeeding women, or individuals sensitive to caffeine.” Consequently, an SFCI must also be added to the product’s principal display panel.
Health Canada offers a variety of SFCI layouts in English, French, and bilingual versions. The size and layout of the SFCI must correspond to the size of the principal display panel. Manufacturers and importers must ensure that their identifiers meet the correct size requirements for the given surface area and the standardized manner of declaration.

List of Permitted Supplemental Ingredients
The List of Permitted Supplemental Ingredients contains ingredients that Health Canada has evaluated and approved for use in supplemented foods. During the TMA period, data collected from various supplemented foods were analyzed to ascertain the safety of these ingredients and establish their conditions of use. Manufacturers can consult this list to verify if a supplemental ingredient can be added to their product, their permissible maximum levels, and the applicable conditions. Additionally, certain ingredients may require a caution statement, with the specific conditions for such statements detailed in this list.
Ingredients not included on the list can be added by submitting a request to Health Canada, accompanied by supporting evidence. Likewise, any requests to modify the current list must be substantiated with appropriate evidence.
The current list includes the following ingredients:
- Vitamins: Biotin, choline, niacin, pantothenic acid, riboflavin, thiamine, beta-carotene, retinol, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E.
- Minerals: Calcium, chromium, copper, iodine, magnesium, molybdenum, phosphorus, potassium, selenium, zinc.
- Amino Acids: L-alanine, l-arginine, l-asparagine, l-aspartic acid, l-cysteine, l-glutamic acid, l-glutamine, glycine, l-histidine, l-isoleucine, l-leucine, l-lysine, l-methionine, l-phenylalanine, l-proline, l-serine, l-threonine, l-tyrosine, l-valine.
- Other Ingredients: Caffeine, green tea extract, phosphatidylserine, taurine.
Key Takeaways
- Supplemented Foods: A new category introduced in Canada, requiring compliance by December 31, 2025.
- Regulatory Framework: Detailed conditions for supplemented ingredients and food categories were established, removing the need for pre-market assessment.
- Manufacturer Responsibilities: Ensure all regulations, including labelling and ingredient use, are met by the compliance deadline.
- Transition from TMA: Products with Temporary Market Authorization must align with the new regulations by the deadline.
- Labelling Requirements: Supplemented foods must include a Supplemented Food Facts Table (SFFt) and, if applicable, a Supplemented Food Caution Identifier (SFCI).
- Permitted Ingredients: Health Canada provides a list of approved supplemental ingredients, with a process for adding or modifying entries.
Summary
The newly established Supplemented Food Regulations offer an innovative path forward, providing oversight for products that did not have their own category under the Food and Drug Regulations. Unlike the Natural Health Products and Temporary Market Authorization routes, the Supplemented Food regulations are more robust and generally do not require a pre-market assessment. The responsibility for compliance lies with the manufacturer, with products regulated through post-market monitoring by the Canadian Food Inspection Agency (CFIA).
While the published list of supplemental ingredients does not encompass many of those reviewed during the TMA period, a process is available for interested parties to request the review and inclusion of additional ingredients.
For questions about Supplemented Foods and label reviews, contact our team! We provide support with label reviews, compliance, and translations. Learn more about the services that we offer for food products on our service page.
Ready to master the new Supplemented Foods regulations?
Strengthen your compliance toolkit with expert-led training from the Dell Tech Academy. Dive into specialized courses covering labelling requirements, SFFt and SFCI implementation, ingredient listing, and seamless transition from TMAs.
👉 Explore our Academy courses here
Dell Tech has provided professional, confidential consulting services to the specialty chemical
industry in Canada, the USA, Europe, and Asia for the last 40 years.





