Unlocking Safety: The Science Behind Irritection® Assay System
By: Dana Mladin, SENIOR TECHNICAL CONSULTANT, email
In today’s world, where safety and innovation go hand in hand, ensuring the products we use every day are gentle on our skin and eyes is paramount. But how do we know which chemicals and formulations are safe without subjecting animals to testing? Enter the Irritection® Assay System, a cutting-edge solution that revolutionizes safety testing while prioritizing ethical practices.
Dell Tech is now the exclusive Canadian distributor of the Irritection® Assay System, and we also perform the testing in-house.
Continue reading to learn more about these new methods we’re excited to offer!
What is the Irritection® Assay System?
The Irritection® Assay System is a state-of-the-art, quantitative in vitro test method designed to predict ocular and dermal irritancy of chemicals, mixtures, and product formulations. It consists of two key tests: Ocular Irritection® (OECD 496) and Irritection® Dermal. These tests utilize changes in relevant macromolecules to provide accurate predictions of irritancy potential, without the need for animal testing.
Why Choose the Irritection® Assay System?
Since 1990, the Ocular Irritection® assay system has provided significant benefits when compared to the in vivo Draize test method. Additionally, the Ocular Irritection® in vitro assay has completed the validation study to meet the standards as defined by the OECD and UN Globally Harmonized System (UN GHS) for classification and has demonstrated high levels of both sensitivity and reproducibility.
Of additional relevance, the Ocular and Dermal Irritection assay methods can be readily employed to evaluate multiple samples at varying volumes or concentrations. Thus, these tests are extremely useful screening tools that facilitate all stages of raw material selection, formulation development and final product selection.
Ethical and Credible
Ocular Irritection® (OI) is the only 100% non-animal ocular irritancy test with OECD adoption, offering top-level scientific credibility and legal support. Irritection® Dermal (ID) provides GHS accepted results under weight of evidence, ensuring ethical testing practices.
Speed and Cost Efficiency
With the Irritection® Assay System, new products can reach the market faster, typically 1-3 months quicker, and at half the cost compared to traditional methods. Test results are obtainable within 24-48 hours, allowing for rapid decision-making and product development.
Versatility
Serving industries including cosmetics, chemicals, personal care, surfactants, and adhesives for over three decades, both OI and ID are valuable tools for achieving compliance with global harmonized system (GHS) regulations.
Quantitative and Reproducible
The Irritection® assays offer quantitative and highly reproducible results, enabling accurate comparative ranking of samples and formulations. Prior studies have demonstrated correlation rates as high as 90% with standard Draize tests and GHS testing.
How Does the Test Work?
The proprietary Ocular and Dermal Irritection® assays are standardized and quantitative in vitro acute ocular and dermal irritation tests. They utilize changes of relevant macromolecules to predict acute ocular and dermal irritancy of chemicals and chemical formulations.
Ocular Irritection® Assay System
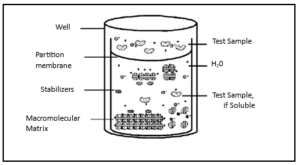
The Ocular Irritection® Assay mimics biochemical phenomena related to corneal irritancy. It involves controlled mixing of the test material with a proprietary reagent solution containing proteins, glycoproteins, lipids, and low molecular weight components. Changes in turbidity (OD405) of the solution indicate alterations in protein structure induced by the test material, allowing calculation of an “irritancy score” directly related to potential human corneal irritancy.
The Ocular Irritection® assay, adopted by OECD TG 496, is a standardized and quantitative in vitro test which utilizes changes of relevant macromolecules to accurately predict acute ocular irritancy of chemicals and chemical formulations. This assay, depicted schematically in Figure 1 below, is based on the principle that chemical compounds will promote measurable changes in target biomolecules and macromolecular structures.
Previous studies clearly demonstrate that the processes of protein denaturation and disaggregation induced in this in vitro assay mimic the effects that are produced when the same types of irritants contact the human eye. Consequently, this in vitro test may be employed to predict in vivo toxic effects of chemicals and formulations.
Figure 1. The Ocular Irritection Model
Dermal Irritection® Assay System
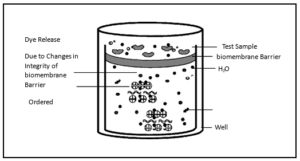
This assay mirrors biochemical changes associated with dermal irritation. A membrane substrate, modified with keratin, collagen, and an indicator dye, is exposed to the test material. Disruption of keratin and collagen structure, along with changes in conformation of globular proteins in the reagent solution, are quantified by measuring optical density changes (OD450). These measurements yield an “irritancy score” directly correlated to potential dermal irritancy.
The Dermal Irritection® assay, depicted schematically in Figure 2, is also based on the principle that chemicals and chemical formulations will promote measurable changes in target biomolecules and macromolecular structures. Previous studies clearly demonstrate that the processes of protein denaturation and disaggregation induced in this in vitro assay mimic the effects produced when the same types of irritants contact the human skin.
Consequently, this in vitro test may be employed to predict the in vivo toxic effects of chemicals and formulations. By application of GHS weight of evidence reasoning, Dermal Irritection® delivers GHS regulatory level results.
Figure 2. The Dermal Irritection Model
In conclusion, the Irritection® Assay System represents a breakthrough in safety evaluation, offering fast, cost-effective, and ethical alternatives to traditional animal testing methods. By harnessing the power of innovative technology, we can continue to prioritize safety without compromising on efficacy or ethical standards.
Talk to our team today to learn more about how the Irritection® Assay System can benefit your company’s product development, hazard classification, shipping and more.