GHS Moving to Version 7 in Canada – What can we expect?
By: Kirsten Alcock, Manager of Product Safety, email
Earlier this month, Health Canada advised that we will be moving from Version 5 to Version 7 of the Globally Harmonized System (GHS) here in Canada. Currently, if you have any questions or comments about the information that was provided in the Canada Gazette, you are encouraged to contact Health Canada directly with your inquiry. My previous blog described how to go about doing this. Please refer to it for more details.
I will be focusing on different classifications that are of importance for my current clients, looking at the currently required statements for Version 5 and determining which classifications will have an impact while moving to Version 7. Today I will focus only on Health Hazard Classifications.
**Please note that if you are within the United States, you are currently on Version 3 of the GHS. You were permitted to use later versions of the GHS if the statements did not contradict or cast doubt on Occupational Safety and Health Administration (OSHA) required information as per the OSHA INSTRUCTION Directive dated July 9, 2015. If you did use Version 3 statements, your labels will need to be redone when OSHA decides to move to later versions. I will write more on that in other blogs once the United States takes steps forward. At this point in time, OSHA has no dates for adoption.
In looking through the Version 7 statements, there do not appear to be any differences in the Health Category statements for Canadian companies who are already in compliance with the GHS Version 5. The statements where we can expect changes are in the Physical Categories. Big changes for Aerosols will be happening so I will write more in the future on this. If you currently sell Aerosol products to workplaces in Canada, I would not suggest printing many labels at this point in time as your label will likely need a change. Contact our lab services team if you need to have your aerosol products tested.
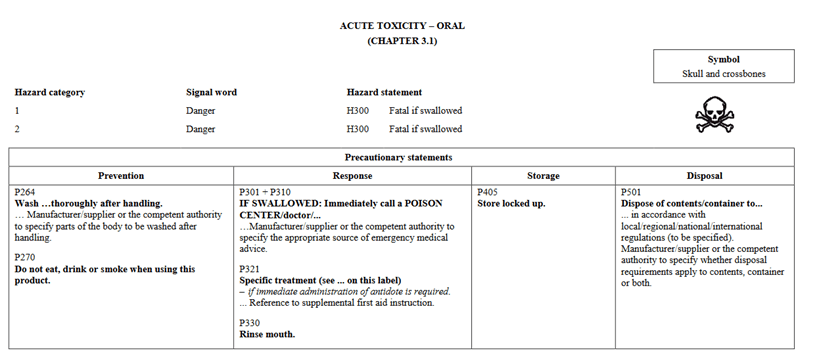
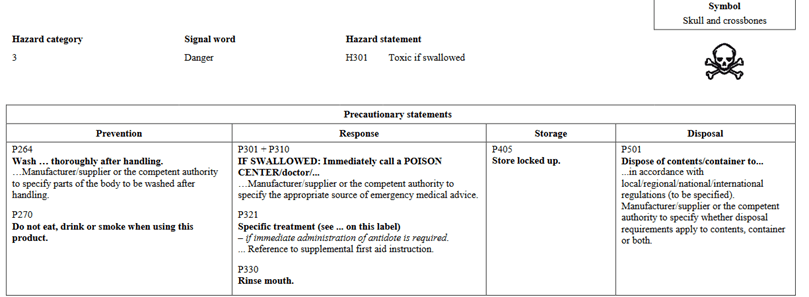
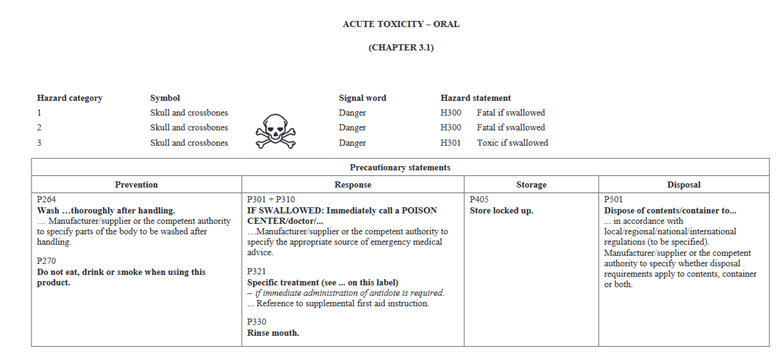
In going through the health categories, I will note that there is a slight change in how information is presented. In Version 5, Acute Toxicity indicated Hazard Category 1 and 2 on the same chart and further separated would you find 3 and 4. In the new GHS Version 7, Categories 1, 2, and 3 are shown in the same table for oral. The phrases are still the same as in the past. They are presented differently. Below I have pictures showing Version 5 vs Version 7 for Oral toxicity.
GHS Version 5:
GHS Version 7:
As far as we know, Canada still has no intention of adopting Category 5 for oral, dermal, or inhalation. Although you will find these statements present in the UN regulations, Canada has not adopted them.
It is very important to understand that countries have a choice. They can choose to adopt GHS or not. They can choose which building blocks they want to adopt. They decide what is best for their country. This is why there is NO GLOBALLY HARMONIZED Safety Data Sheet (SDS).
Each country can choose what they want to adopt therefore you must ensure that the SDS you are using is appropriate for the country you are selling to.
If you would like further information on your safety data sheet requirements for Canada or the United States or are interested in updating your existing documents to be in compliance with the appropriate regulatory requirement, please contact us. We’d be happy to help you with the process.
Contact:
Dell Tech
Kirsten Alcock, B.Sc. (Hons)
Manager, Product Safety Group
519-858-5074
kirsten@delltech.com
Dell Tech has provided professional, confidential consulting services to the chemical specialty
industry in Canada, the USA, Europe, and Asia for the last 40 years.
Contact us today for more information.